
Selective Elimination of Senescent Cells (SnCs): ApoptiCIDe-CE-GERO™
-
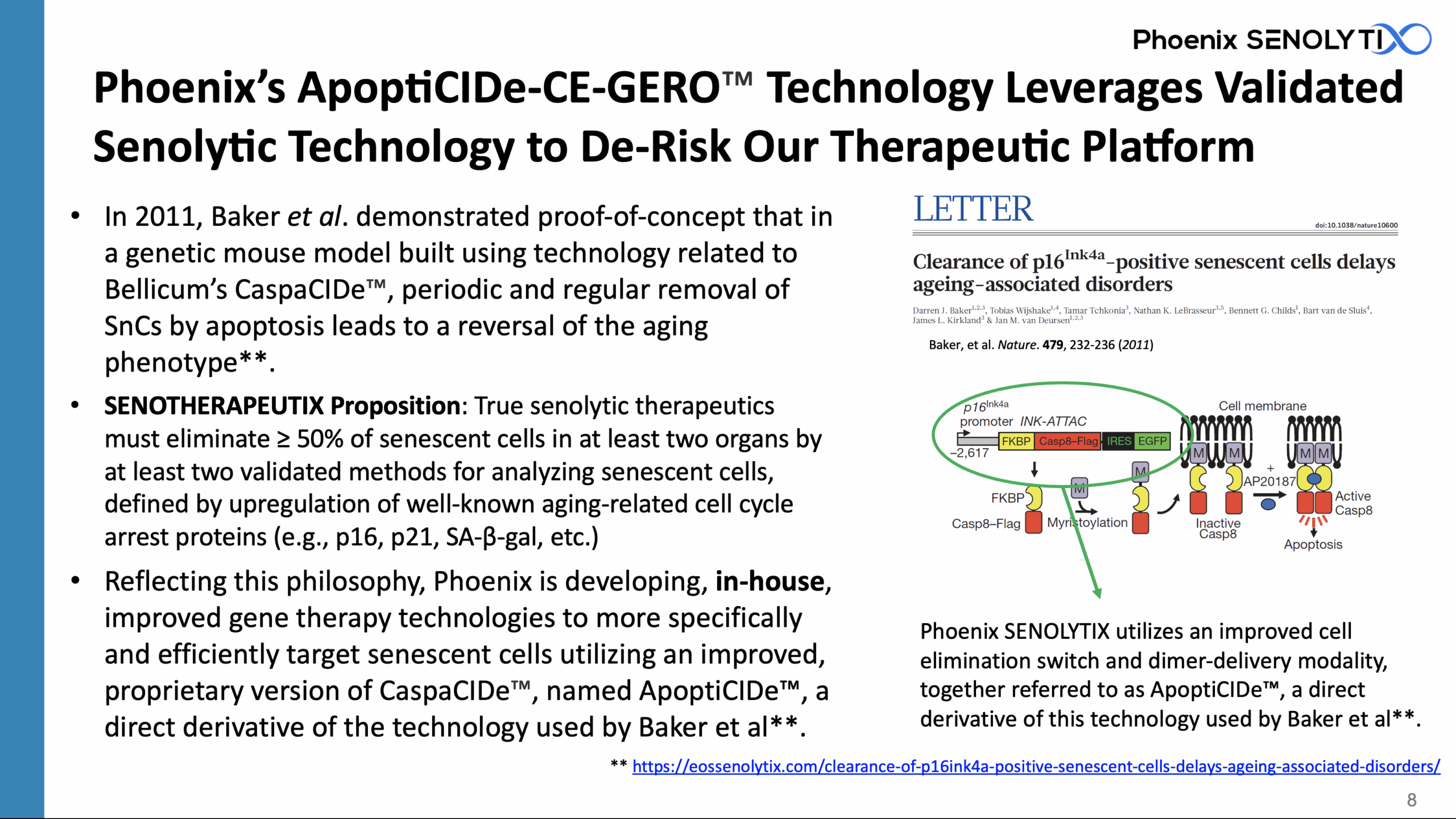
In 2011, Baker et al** demonstrated proof-of-concept that in a genetic mouse model built using technology owned by Bellicum Pharmaceuticals, Inc. and related to Bellicum’s CaspaCIDe™, periodic and regular removal of SnCs by dimerizer-induced apoptosis led to reversal of the aging phenotype
-
Phoenix SENOLYTIX is developing, in-house, gene therapy technologies to more specifically and efficiently target senescent cells for elimination utilizing an improved, proprietary version of CaspaCIDe™, named ApoptiCIDe™, a direct derivative of the technology used by Baker et al**.
-
SENOTHERAPEUTIX Proposition: True senolytic therapeutics must eliminate ≥ 50% of senescent cells in at least two organs by at least two validated methods for quantifying senescent cells, defined by upregulation of well-known aging-related cell cycle arrest proteins (e.g., p16, p21, SA-β-gal, etc.)
** https://eossenolytix.com/clearance-of-p16ink4a-positive-senescent-cells-delays-ageing-associated-disorders/
ApoptiCIDe-CE-GERO-002™
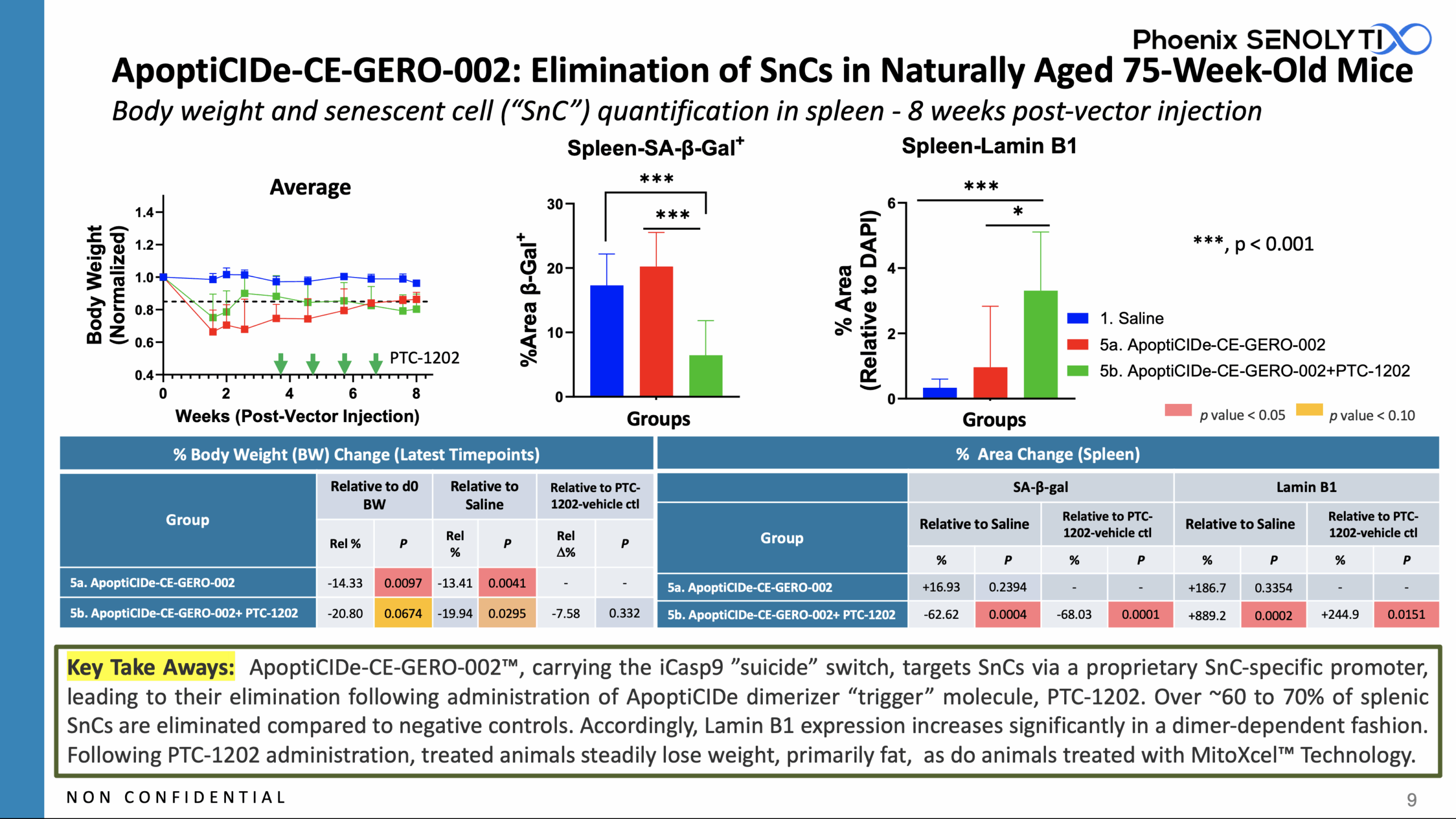
ApoptiCIDe-CE-GERO-002™ Eliminates ~ 60% to 70% of SnCs With a Single Dose of Vector in Naturally Aged 75-Week-Old Mice
-
ApoptiCIDe-CE-GERO-002™, carrying the iCasp9 ”suicide” switch, targets SnCs via a proprietary SnC-specific promoter, leading to their elimination following administration of the ApoptiCIDe dimerizer “trigger” molecule, PTC-1202.
-
PTC-1202 is Phoenix’s proprietary injectable formulation of rimiducid, the small molecule activator of the ApoptiCIDe™️ cell elimination switch
-
2.5E11 vg of ApoptiCIDe-CE-GERO-002™ was injected intraperitoneally (IP) into groups of natuarlly aged 75 week old C57Bl/6 female mice on Day 0. Group 5a received vector only, but in Group 5b, PTC-1202, the ApoptiCIDe “trigger” was administered weekly for four doses beginning ~ 4 weeks after vector administration.
-
Over ~60 to 70% of splenic SnCs were eliminated in ApoptiCIDe-CE-GERO-002™ + PTC-1202-treated animals compared to animals that did not receive PTC-1202 and to saline treated negative controls. Accordingly, Lamin B1 expression also increased significantly in a dimer-dependent fashion.
-
Following PTC-1202 administration, treated animals steadily lose weight, primarily fat, as do animals treated with MitoXcel™ Technology.
Selective Elimination of White Adipose Tissue (WAT): ApoptiCIDe-CE-WAT™
ApoptiCIDe-CE-WAT-001™: WAT-Specific In Vivo Adipocyte Elimination
-
ApoptiCIDe-CE-WAT-001™️ vector was administered IP as indicated at a dose of 2.5E11 vg on Day 0
-
SMG or PTC-1202 was administered as indicated beginning on Day 16
-
PTC-1202 is Phoenix’s proprietary injectable formulation of rimiducid, the small molecule activator of the ApoptiCIDe™️ cell elimination switch
-
Treatment with ApoptiCIDe-CE-WAT™️ + PTC-1202 led to 14.6% lower weight compared to saline-treated animals and an 8.5% lower weight compared to SMG treated animals.
-
Upon discontinuation, the rebound in body weight gain was greater after SMG withdrawal that after PTC-1202 withdrawal.
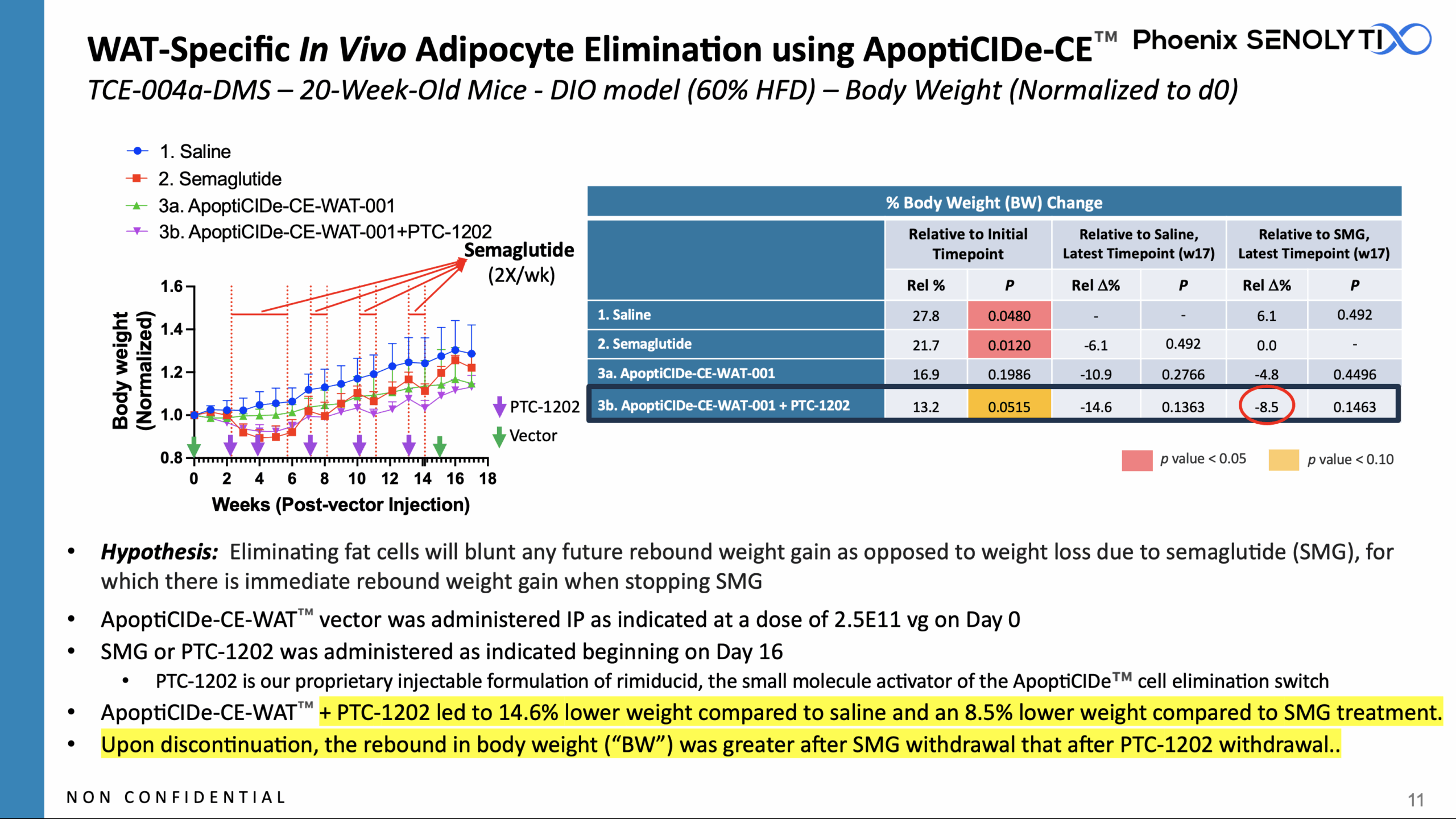
ApoptiCIDe-CE-WAT-001™: WAT-Specific In Vivo Adipocyte Elimination
ApoptiCIDe-CE-WAT-001™ Eliminates More Fat More Quickly Than Semaglutide, While Increasing Lean Mass
-
ApoptiCIDe-CE-WAT-001™, carrying the iCasp9 ”suicide” switch, targets the adipocytes in white adipose tissue, via a proprietary WAT-specific promoter.
-
DEXA scan shows a 55% greater reduction in fat, with a concomitant 4% greater increase in lean mass, in ApoptiCIDe-CE-WAT-001™ + PTC-1202-treated animals compared to the saline-treated group.
-
DEXA scan also showed a 54% greater reduction in fat, with a concomitant 5% greater increase in lean mass, in ApoptiCIDe-CE-WAT-001™ + PTC-1202-treated animals compared to the SMG-treated group
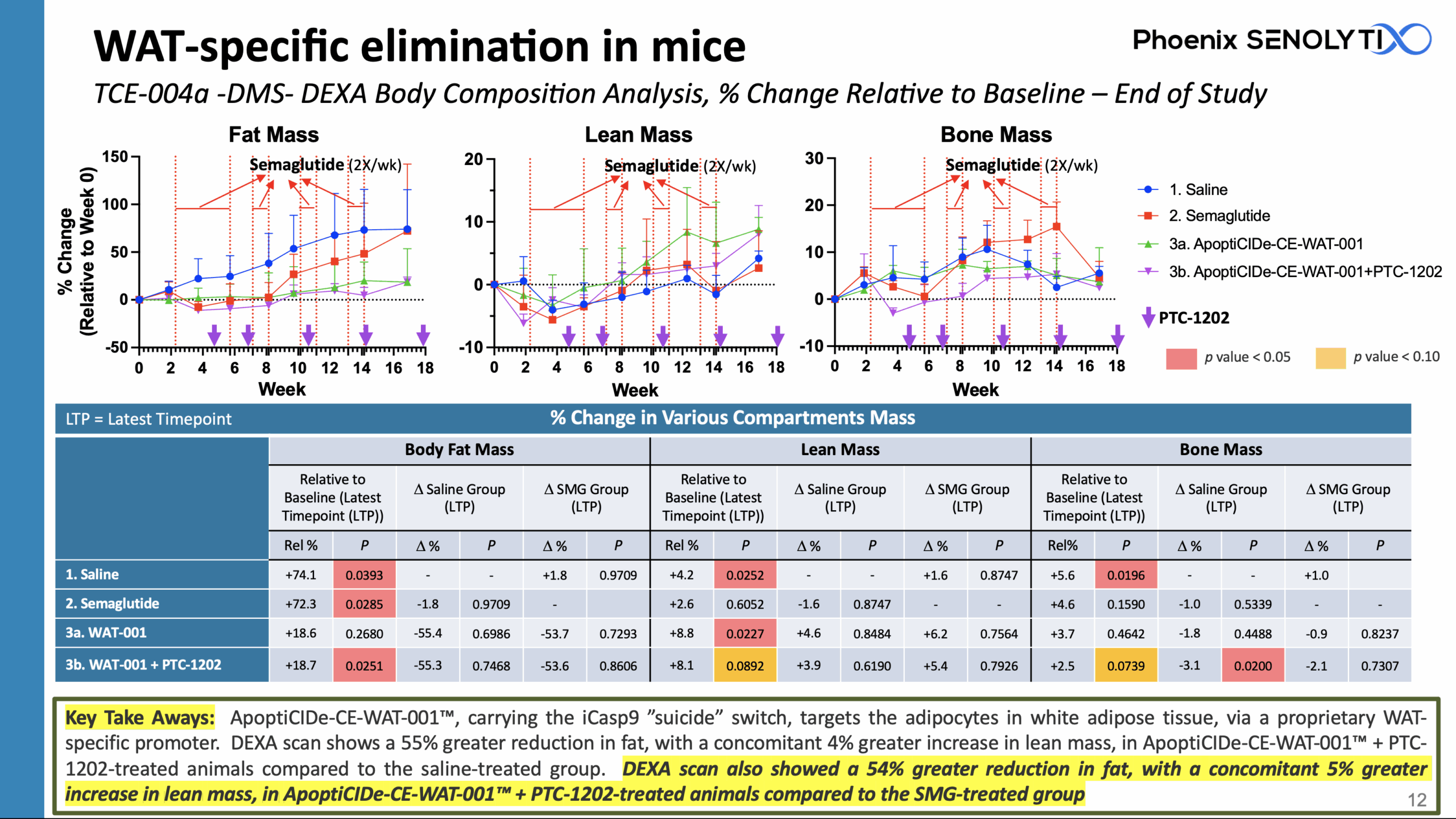
ApoptiCIDe-CE-WAT-001™: WAT-Specific In Vivo Adipocyte Elimination
ApoptiCIDe-CE-WAT-001™ Eliminates White Adipose Tissue (WAT), While Avoiding Brown Adipose Tissue (BAT)
-
ApoptiCIDe-CE-WAT-001™, carrying the iCasp9 ”suicide” switch, targets the adipocytes in white adipose tissue, via a proprietary WAT-specific promoter, leading to their elimination following administration of ApoptiCIDe dimerizer “trigger” molecule, PTC-1202.
-
Expression appears to be limited to WAT over BAT.
-
In ex-vivo harvested inguinal adipose tissue (IAT), there was a PTC-1202-dependent ~35% decrease in BLI in the ex vivo IAT in animals that received PTC-1202 as compared to those that didn’t.
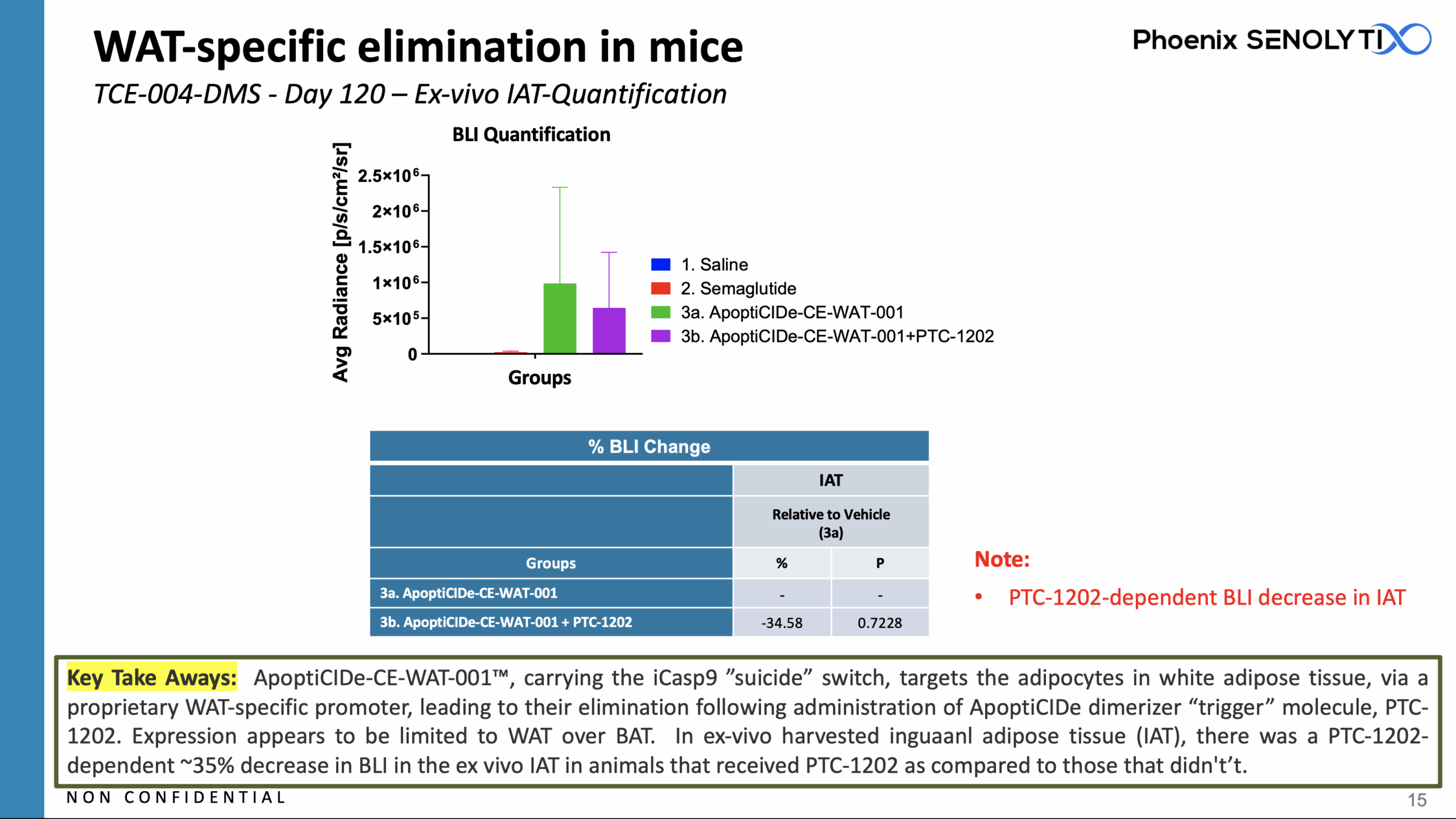
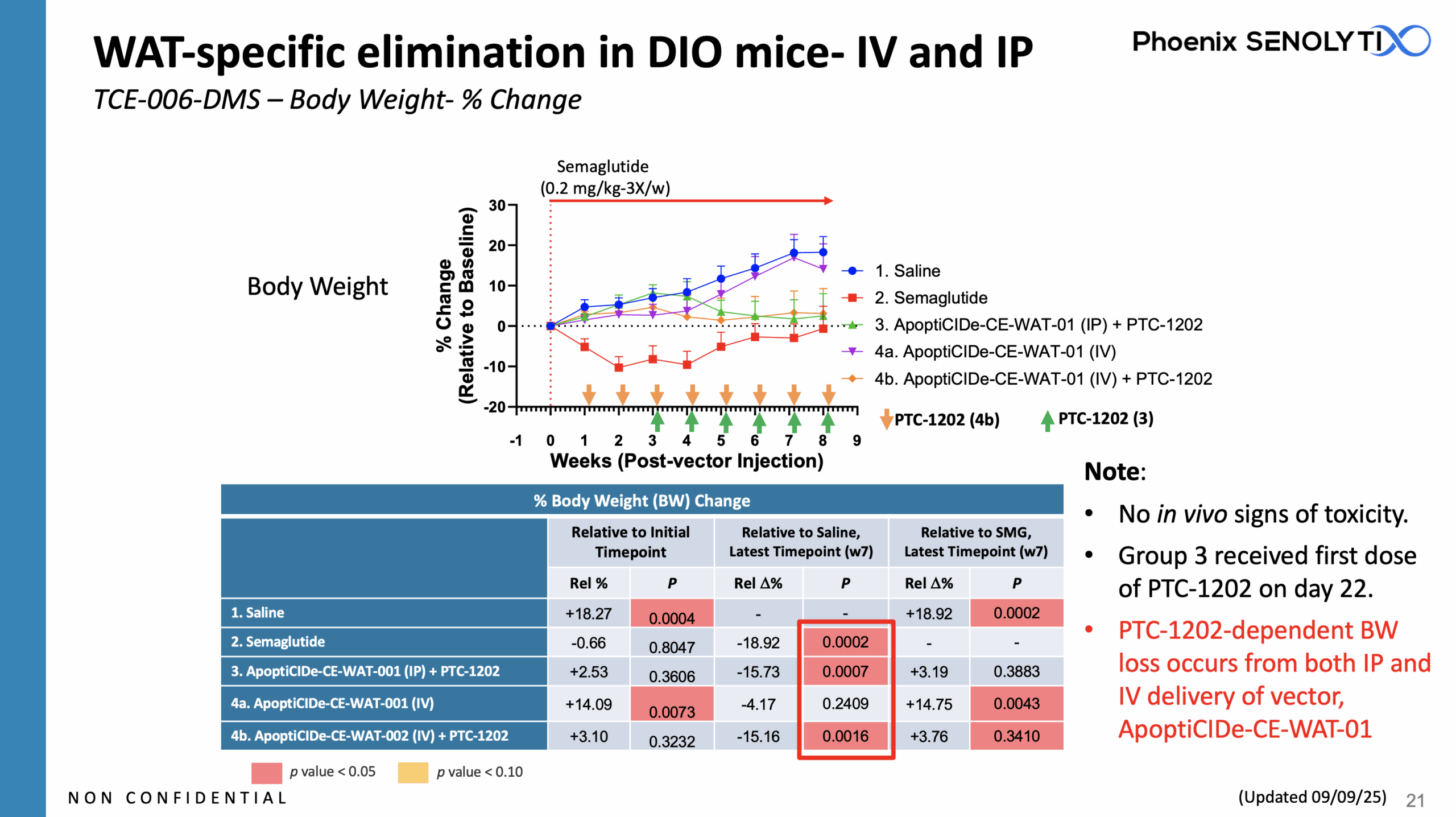
ApoptiCIDe-CE-WAT-001™: WAT-Specific Elimination in DIO Mice: IV and IP
ApoptiCIDe-CE-WAT-001™ Eliminates White Adipose Tissue (WAT) When Delivered Both IP and IV
-
ApoptiCIDe-CE-WAT-001™, carrying the iCasp9 ”suicide” switch, targets the adipocytes in white adipose tissue, via a proprietary WAT-specific promoter, leading to their elimination following administration of ApoptiCIDe dimerizer “trigger” molecule, PTC-1202.
-
Equivalent PTC-1202-dependent Body Weight (“BW”) loss occurs from both IP and IV delivery of vector, ApoptiCIDe-CE-WAT-01.
-
There were no in vivo signs of toxicity.
ApoptiCIDe-CE-WAT-001™: WAT-Specific Elimination in DIO Mice: IV and IP
IV Administered ApoptiCIDe-CE-WAT-001™ Eliminates More Fat and Preserves More Lean Mass Than In Semaglutide (“SMG”) Treated Animals
-
ApoptiCIDe-CE-WAT-001™, carrying the iCasp9 ”suicide” switch, targets the adipocytes in white adipose tissue, via a proprietary WAT-specific promoter, leading to their elimination following administration of ApoptiCIDe dimerizer “trigger” molecule, PTC-1202.
-
When ApoptiCIDe-CE-WAT-001™ is delivered IV, DEXA scan shows a 42% greater reduction in fat (p=0.036) after administration of the activating agent, PTC-1202, compared to the saline-treated group and a 13% greater reduction in fat compared to the SMG-treated group.
-
In IV treated animals, DEXA scan also showed a 2% greater increase in bone mass after administration of PTC-1202 compared to saline treated animals, and a 3.5% greater increase in lean mass compared to SMG-treated animals, who expectedly lost 5.4% of lean mass compared to saline-treated control animals


